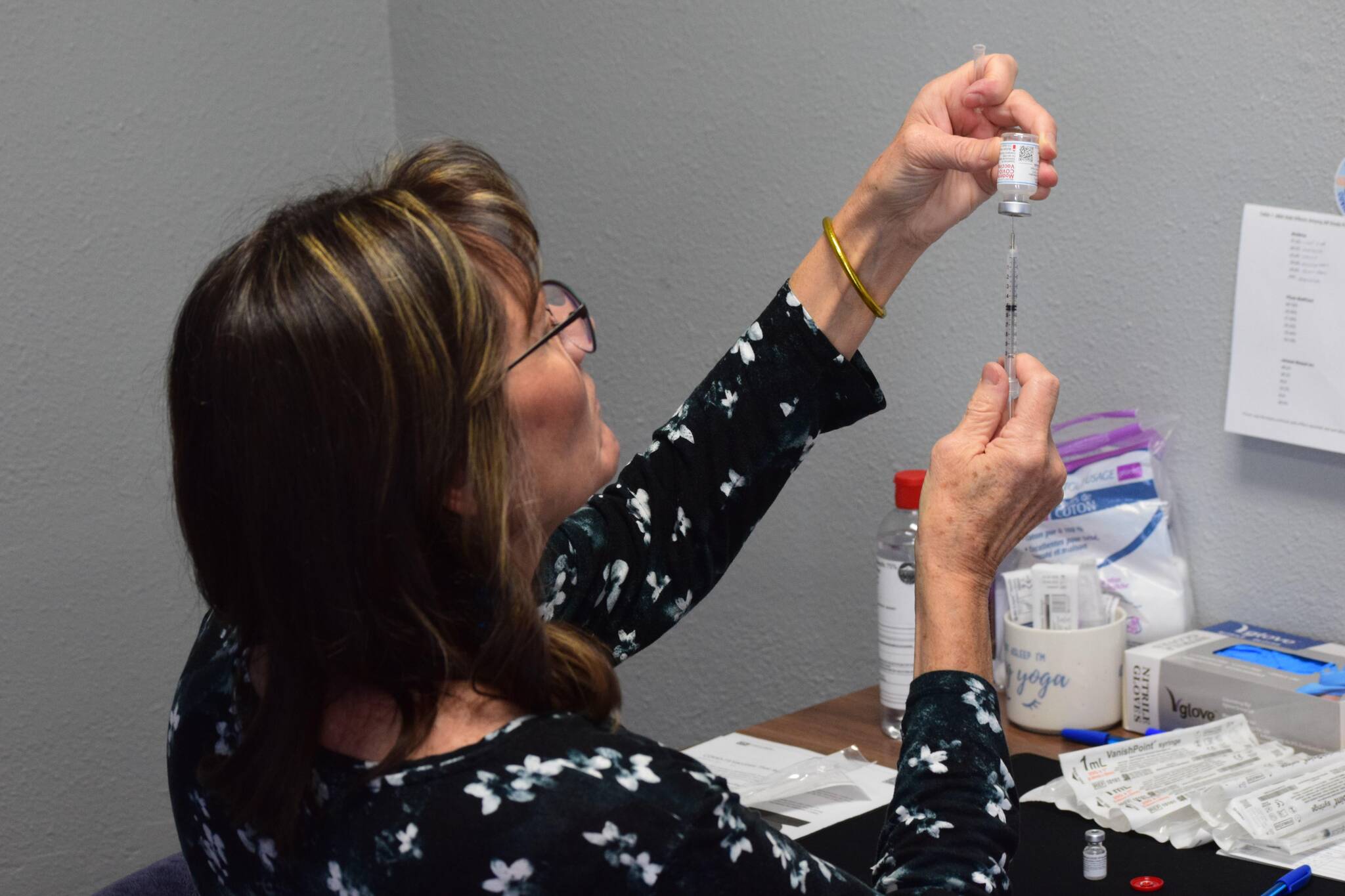
COVID-19 vaccines for kids younger than 5 years old are now approved by both the Food and Drug Administration and the Centers for Disease Control and Prevention, and doses are already on the central peninsula.
Tami Marsters, a nurse with Kenai Public Health, said last Friday the clinic is offering approved Moderna vaccines to kids ages 6 months to 5 years old. This comes less than a week after the CDC gave the final go-ahead for the shots.
In Homer, parents seeking COVID-19 vaccines for children younger than 5 should visit local medical clinics, South Peninsula Hospital Public Information Officer Derotha Ferraro told the Homer City Council at its meeting on Monday.
According to the Associated Press, the advancement came earlier this month after advisers to the CDC recommended the vaccines to younger children. The AP reported roughly 18 million kids nationwide will now be eligible to get their COVID vaccines.
As of last week, Soldotna Professional Pharmacy, the Kenai Walmart, and Walgreens were still only offering COVID vaccines to those 5 and older. Last Wednesday, 65.4% of Alaskans 5 and up had completed their primary vaccine series, according to state data.
Officials recommend all eligible Alaskans be up to date on their COVID vaccines to minimize the infection’s impact on communities.
The Pfizer-BioNTech and Moderna shots are approved for anyone 6 months or older. Booster shots are recommended, whether or not a person has already contracted the virus and despite elapsed time since the completion of the primary series.
The FDA said the Johnson & Johnson/Janssen shot should only be given to adults who cannot receive a different vaccine or specifically request J&J’s vaccine, according to the AP. U.S. authorities for months have recommended that Americans get Pfizer or Moderna shots instead of J&J’s vaccine, the AP reported.
The FDA and CDC are recommending Pfizer boosters for anyone 12 and older at least five months after the primary series. Additionally, Moderna boosters are recommended for anyone 18 and older at least six months after a primary series.
For those 50 years and older who are up to date with their primary series and first booster, another dose of either Pfizer or Moderna is authorized four months after the initial booster dose. In this category, a person with three vaccines of any combination of Pfizer or Moderna is now eligible for a fourth dose, and those with a single Janssen shot and booster can now receive a third dose of either Pfizer or Moderna.
In addition, certain immunocompromised individuals can also receive another Pfizer or Moderna shot four months after their last booster. This would include three shots for a primary series and two additional booster doses.
A map of vaccine providers can be found on DHSS’ COVID-19 vaccine website at covidvax.alaska.gov.
Reach reporter Camille Botello at camille.botello@peninsulaclarion.com.